Abstract
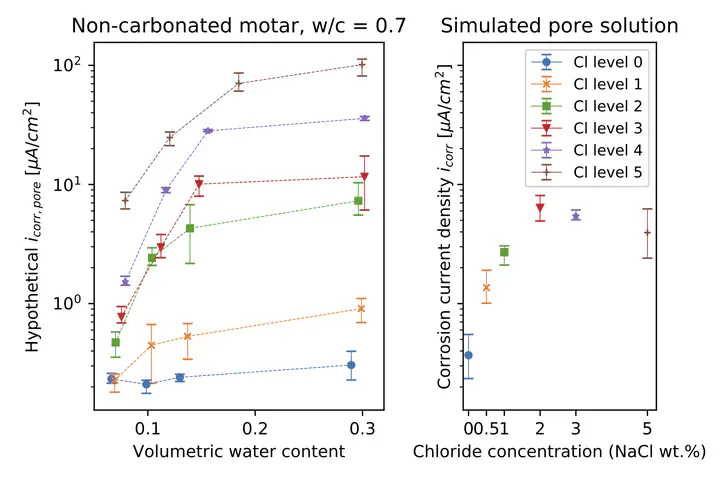
This study investigates the synergistic effects of chloride ions, carbonation, and relative humidity on rebar corrosion in mortar, elucidating the complex interactions at the rebar-mortar interface. Findings reveal that chloride ions and carbonation significantly alter the volumetric water content and resistivity of mortar, leading to non-binary corrosion behaviour through area effect, ohmic control, and anode-to-cathode ratio. In chloride-free, non-carbonated mortar, the area effect dominates, whereas the presence of chloride introduces ohmic control and a saturation-dependent anode-to-cathode ratio. In carbonated mortar, a distinctive ohmic control mechanism emerges, governed by local ionic conductivity. This study highlights the critical need to consider these combined effects for accurate prediction and mitigation of rebar corrosion in concrete structures. By advancing the understanding of these mechanisms, the research provides valuable insights for improving the durability of reinforced concrete structures exposed to harsh environmental conditions.