Abstract
Corrosion of reinforced concrete (RC) structures is a complex problem, and corrosion rate prediction is a challenging task. The impact of cracking on corrosion rates during the propagation phase is influenced by concrete properties and further complicated by moisture content fluctuation, chloride ion contamination and carbonation in the cracked reinforced concrete structures.
Numerical simulation is a versatile tool that allows an in-depth assessment of corrosion of RC structures. However, current numerical models face challenges in describing the corrosion cell of rebars in concrete in the presence of spatial variations of microenvironment due to cracking and service exposure conditions.
The objective of this research was to develop a comprehensive mechanistic model for predicting the corrosion rate of cracked concrete in realistic service environments. In this model, the homogenized corrosion kinetics of rebar were coupled with the evolution of the non-uniform microenvironment in concrete caused by cracking of concrete and varying exposure conditions. This objective was achieved by conducting a series of experimental programs and numerical simulations.
The first-phase experimental program quantified the corrosion parameters of rebar such as corrosion potential, corrosion current density and Tafel slopes in simulated pore solutions and mortar with various chloride content, carbonation, and moisture conditions. A novel two-step potentiodynamic test routine was developed, and great care was taken to ensure a realistic rebar condition history such as preserving the mill scale, passivation of rebar prior to exposure to the corrosive environments, and extended exposure periods.
The experimental results revealed the synergistic effect of chloride, moisture, and carbonation on the corrosion parameters of rebar. It was found that the role of water saturation on corrosion includes 1) an area effect, which affects the rebar-pore solution area, 2) an ohmic control effect by changing the bulk resistivity, and 3) an anode-to-cathode ratio effect by altering the anodic and cathodic site area ratio. These effects complicate the role of chloride and carbonation on rebar corrosion in mortar, rather than being solely dependent on chloride concentration and pH in simulated pore solutions.
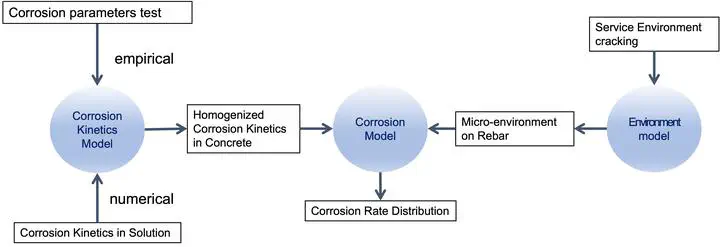
The first-phase study provided a database, from which the corrosion kinetics model was developed, where the homogenized corrosion parameters were empirical functions of chloride content, water saturation, and carbonation. In addition, it provided the basis for modelling the corrosion kinetics in concrete from corrosion in solution.
In the modelling phase, a circuit model was developed to simulate the polarization behaviour of a rebar experiencing non-uniform surface conditions. It started from localized corrosion kinetics for active and passive rebar-concrete units (mesoscopic–macroscopic level) and arrived at a simulated scan curve representing the overall performance of the rebar, from which the homogenized corrosion parameters were derived. The simulation results confirmed the hypothesis that the homogenized corrosion parameters are functions of concrete resistivity and the active-to-passive rebar-concrete interface area ratio.
Building upon the circuit model, a simulation approach was developed to derive the homogenized corrosion parameters of the rebar-concrete interface (mesoscopic–macroscopic level) from the corrosion kinetics of the rebar-solution interface (microscopic pore level). It featured in describing the allocation of active and passive rebar-solution corrosion sites in the representative elementary volume based on the non-uniform water and chloride distribution in concrete pores. The simulated homogenized corrosion kinetics were functions of the total chloride content and water saturation of concrete, and they captured the patterns of the empirical functions from the first-phase experimental study.
To simulate the microenvironment evolution at the rebar depth of a concrete structure, a comprehensive 3-D transport model (environment model) was developed. In the case study of an uncracked RC structure — the University Bridge subjected to service conditions with the source of chloride, carbonation, varying relative humidity, and exposure to unevenly sheltered run-off water, this model predicted the corrosion rates of century-old rebar in concrete by accurately modelling the microenvironment at rebar depth with a realistic depiction of the microclimate as well as various speculated climate scenarios, including elevated CO2 levels and changes in precipitation.
Finally, a comprehensive corrosion model was formulated by incorporating crack configuration into the environment model and coupling it with the corrosion kinetics model. This model simulated the transport of species such as water, chloride ion, oxygen, and carbon dioxide in the concrete domain and the electrochemical reaction on the rebar-concrete interface and solved for the potential and current fields. A second-phase experimental program was designed to validate this model, using segmented rebars in cracked concrete specimens subjected to wetting and drying cycles with chloride salt solutions.
The validated model enabled a numerical investigation of the mechanisms behind varying field observations of rebar corrosion in cracked concrete. It explained the complex effects of cracks on corrosion, including corrosion reduction over time, crack size and healing effects.
Under submerged conditions, cracks affect corrosion by affecting the diffusion rate of chloride ions through the crack. Thinner cracks lead to less chloride influx, resulting in a reduced peak corrosion current in the cracking zone. The corrosion rate decreases over time and eventually reaches a similar level limited by oxygen diffusion that is independent of crack size.
Under wetting and drying conditions, the corrosion kinetics of rebar in the cracking zone are affected by chloride and moisture, with the possibility of rebar acting as a cathode during drying cycles. The average corrosion rate in the cracking zone peaks during wetting cycles and decreases during subsequent drying cycles, with the peak values diminishing with repeated wetting and drying cycles. Micro-cell corrosion eventually becomes the primary contributor to rebar corrosion in the cracking zone and the rate depends on the chloride content on the rebar and oxygen concentration.
Crack healing results in reduced transport properties, dilution of high chloride content near the crack, and limited oxygen diffusion. It accelerates corrosion rate reduction during wetting and drying cycles. Less permeable concrete, such as high performance concrete, benefits more from crack healing with greater chloride dilution. The critical crack width below which it is considered harmless should be determined based on the effects of the crack on corrosion with specific material properties and exposure conditions.